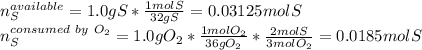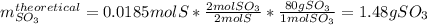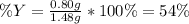Chemistry, 20.03.2020 00:28, madisonenglishp2qkow
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollutant: 2S(s) + 3O2(g) → 2SO3(g) In a particular experiment, the reaction of 1.0 g S with 1.0 g O2 produced 0.80 g of SO3. The % yield in this experiment is .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3


Do you know the correct answer?
Sulfur and oxygen react in a combination reaction to produce sulfur trioxide, an environmental pollu...
Questions in other subjects:



Mathematics, 16.01.2020 01:31



Mathematics, 16.01.2020 01:31




Biology, 16.01.2020 01:31