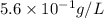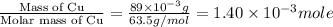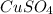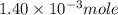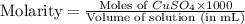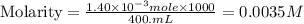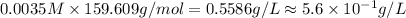Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control ch...

Chemistry, 20.03.2020 00:39, xocupcake309174
Fe(s) + CuSO4(aq) <===> Cu(s) + FeSO4(aq)
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 400.mL copper (II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 89.mg .
Calculate the original concentration of copper (II) sulfate in the sample. Round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, annarain2004
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1


Do you know the correct answer?
Questions in other subjects:

Mathematics, 23.09.2021 09:50


History, 23.09.2021 09:50

Advanced Placement (AP), 23.09.2021 09:50


Mathematics, 23.09.2021 09:50


Chemistry, 23.09.2021 09:50


Health, 23.09.2021 09:50