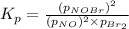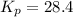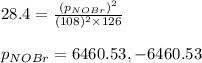Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
Do you know the correct answer?
Consider the reaction: 2 NO(g) + Br2(g) ∆ 2 NOBr(g) Kp = In a reaction mixture at equilibrium, the p...
Questions in other subjects:

Mathematics, 04.09.2020 02:01

English, 04.09.2020 02:01





Mathematics, 04.09.2020 02:01



Biology, 04.09.2020 02:01


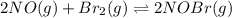
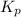 for above equation follows:
for above equation follows: