
Chemistry, 20.03.2020 00:06, buddyclayjohnson
A mixture of methane (CH4) and ethane (C2H6) is stored in a container at 294 mm Hg. The gases are burned in air to form CO2 and H2O. If the pressure of CO2 is 351 mm Hg measured at the same temperature and volume as the original mixture, calculate the mole fraction of the gases.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
Do you know the correct answer?
A mixture of methane (CH4) and ethane (C2H6) is stored in a container at 294 mm Hg. The gases are bu...
Questions in other subjects:



Chemistry, 24.04.2020 15:49





Mathematics, 24.04.2020 15:49



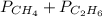 = 294 mm Hg
= 294 mm Hg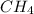 will yield 1 mole of
will yield 1 mole of 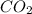 whereas 1 mole of
whereas 1 mole of 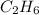 will yield 2 moles of
will yield 2 moles of 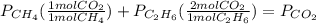
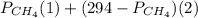 = 351 mm Hg
= 351 mm Hg = 2(294) - 351
= 2(294) - 351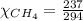
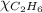 = 1 - 0.806
= 1 - 0.806



