
For the reaction CO2(g) + H2O(ℓ) + CaCl2(aq) ←→ CaCO3(s) + HCl(aq) K = 4.8 the concentrations of CO2, H2O, CaCl2 and HCl are 0.377 M, 55.4 M, 0.652 M and 30.2 M, respectively. What will happen in order for the system to reach equilibrium? 1. The reaction will shift to the right. 2. Nothing will occur. 3. The reaction will shift to the left. 4. Not enough information is given.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Do you know the correct answer?
For the reaction CO2(g) + H2O(ℓ) + CaCl2(aq) ←→ CaCO3(s) + HCl(aq) K = 4.8 the concentrations of CO2...
Questions in other subjects:

Health, 26.07.2019 23:00

English, 26.07.2019 23:00

Mathematics, 26.07.2019 23:00


Business, 26.07.2019 23:00


Mathematics, 26.07.2019 23:00

History, 26.07.2019 23:00


Advanced Placement (AP), 26.07.2019 23:00


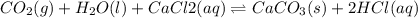
![K=\frac{[HCl]^2}{[CaCl_2]}](/tpl/images/0554/8954/74ccf.png)
![Q=\frac{[HCl]^2}{[CaCl_2]}=\frac{ 30.2^2 }{0.652 }=1398.8](/tpl/images/0554/8954/f4b79.png)




