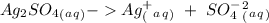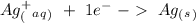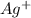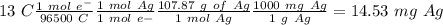Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Do you know the correct answer?
Suppose a current of 250.mA is passed through an electroplating cell with an aqueous solution of Ag2...
Questions in other subjects:


Mathematics, 21.10.2020 21:01








Mathematics, 21.10.2020 21:01