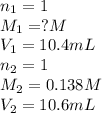Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
Do you know the correct answer?
An aqueous solution of hydroiodic acid is standardized by titration with a 0.138 M solution of potas...
Questions in other subjects:





History, 17.04.2020 18:31


Mathematics, 17.04.2020 18:31


Mathematics, 17.04.2020 18:31


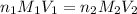
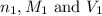 are the n-factor, molarity and volume of acid which is HI
are the n-factor, molarity and volume of acid which is HI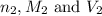 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.