
Chemistry, 19.03.2020 22:34, skatingflower
When the chemical reaction A + B ⇀↽ C + D is at equilibrium, 1. neither the forward nor the reverse reactions have stopped. 2. all four concentrations are equal. 3. both the forward and reverse reactions have stopped. 4. the sum of the concentrations of A and B equals the sum of the concentrations of C and D. 5. the reverse reaction has stopped. 6. the forward reaction has stopped.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
When the chemical reaction A + B ⇀↽ C + D is at equilibrium, 1. neither the forward nor the reverse...
Questions in other subjects:

Mathematics, 08.11.2019 07:31


Physics, 08.11.2019 07:31

Mathematics, 08.11.2019 07:31

Chemistry, 08.11.2019 07:31

Mathematics, 08.11.2019 07:31

Mathematics, 08.11.2019 07:31

Mathematics, 08.11.2019 07:31


Spanish, 08.11.2019 07:31


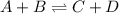
![K=\frac{[C]\times [D]}{[A]\times [B}}](/tpl/images/0554/7763/ae12b.png)




