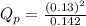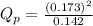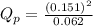Chemistry, 19.03.2020 22:42, Nyasiabaltimore3
He equilibrium constant (KP) is 0.21 at a particular temperature for the reaction:
N2O4(g) <=> 2NO2(g)
Given the following sets of initial conditions, what is the net change that must occur for the reaction to reach equilibrium? Does the reaction shift left to reach equilibrium, does the reaction shift right to reach equilibrium or is the reaction at equilibrium at these initial concentrations so no net change will occur?
Options: Equilibrium, Left, or Right
a. PNO2 = 0.225 atm, PN2O4 = 0.134 atm
b. PNO2 = 0.086 atm, PN2O4 = 0.064 atm
c. PNO2 = 0.130 atm, PN2O4 = 0.142 atm
d. PNO2 = 0.173 atm, PN2O4 = 0.142 atm
e. PNO2 = 0.151 atm, PN2O4 = 0.062 atm

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1
Do you know the correct answer?
He equilibrium constant (KP) is 0.21 at a particular temperature for the reaction:
N2O4...
N2O4...
Questions in other subjects:

World Languages, 22.07.2021 14:00

Social Studies, 22.07.2021 14:00

Business, 22.07.2021 14:00


English, 22.07.2021 14:00

Physics, 22.07.2021 14:00

English, 22.07.2021 14:00





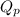 as follows.
as follows.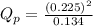
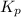 is 0.21 (which is smaller than the value) this means that equilibrium will shift to the left
.
is 0.21 (which is smaller than the value) this means that equilibrium will shift to the left
.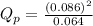
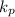 which means that the equilibrium will shift to the right.
which means that the equilibrium will shift to the right.