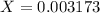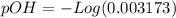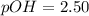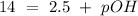Chemistry, 19.03.2020 22:19, nativebabydoll35
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M solution of allantoin at 25°C. Round your answer to 1 decimal pl

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
Do you know the correct answer?
The base protonation constant Kb of allantoin (C4H4N3O3NH2) is ×9.1210−6. Calculate the pH of a 1.1M...
Questions in other subjects:

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Health, 11.09.2020 06:01

Geography, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01

Mathematics, 11.09.2020 06:01


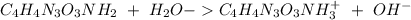
![Kb=\frac{[C_4H_4N_2O_3NH_3^+][OH^-]}{[C_4H_4N_3O_3NH_2]}](/tpl/images/0554/7437/19295.png)
![9.2x10^-^6=\frac{[X][X]}{[1.1-X]}](/tpl/images/0554/7437/d576a.png)
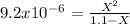
![(9.2x10^-^6)*[1.1-X]=X^2](/tpl/images/0554/7437/e621d.png)