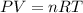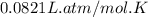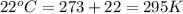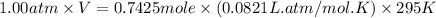Chemistry, 19.03.2020 21:25, xemnas1994
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated, thus inflating the bag.2NaN3(s)⟶2Na(s)+3N2(g)Calculate the value of work, with, for the system if32.2NaNO3 reacts completely at1.00 atmand22∘C.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
Do you know the correct answer?
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated,...
Questions in other subjects:




Mathematics, 17.02.2021 17:30

Mathematics, 17.02.2021 17:30

Mathematics, 17.02.2021 17:30

History, 17.02.2021 17:30

Social Studies, 17.02.2021 17:30

Mathematics, 17.02.2021 17:30


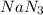
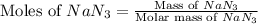
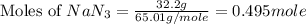
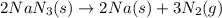
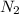
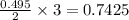 moles of
moles of