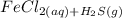Chemistry, 19.03.2020 21:27, Hamadsaqer9
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S and aqueous iron(II) chloride . Write a balanced chemical equation for this reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
Do you know the correct answer?
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S...
Questions in other subjects:

Physics, 18.01.2021 20:10

English, 18.01.2021 20:10



Biology, 18.01.2021 20:10

Social Studies, 18.01.2021 20:10

Mathematics, 18.01.2021 20:10



Chemistry, 18.01.2021 20:10