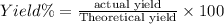Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3
Do you know the correct answer?
When 60.0 g of CH4 reacts with excess O2, the actual yield of CO2 is 112 g. What is the percent yiel...
Questions in other subjects:

Mathematics, 18.11.2020 17:30

Mathematics, 18.11.2020 17:30




Biology, 18.11.2020 17:30


Mathematics, 18.11.2020 17:30



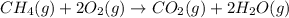

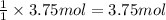 of carbon dioxide gas
of carbon dioxide gas