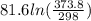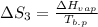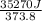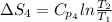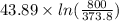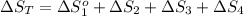The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-mol. Methanol boils at 337K with an enthalpy of vaporization of 35.270 kJ/mol at that temperature. The heat capacity of the vapor is 43.9 J/K-mol.__Calculate the entropy of one mole of methanol vapor at 800 K.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1


Chemistry, 23.06.2019 08:00, sassy11111515
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
Do you know the correct answer?
The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-m...
Questions in other subjects:



Mathematics, 04.02.2020 21:00



Chemistry, 04.02.2020 21:00



Mathematics, 04.02.2020 21:00

Biology, 04.02.2020 21:00


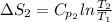 J/K mol
J/K mol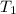 = 298 K,
= 298 K, 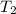 = 373.8 K
= 373.8 K