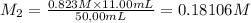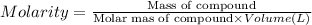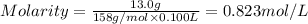Chemistry, 19.03.2020 20:56, Uhmjujiooo45701
By pipet, 11.00 mL of a 0.823 MM stock solution of potassium permanganate (KMnO4) was transferred to a 50.00-mL volumetric flask and diluted to the calibration mark. Determine the molarity of the resulting solution. A stock solution of potassium permanganate (KMnO4) was prepared by dissolving 13.0g KMnO4 with DI H2O in a 100.00-mL volumetric flask and diluting to the calibration mark. Determine the molarity of the solution Molarity= O.822 M

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
Do you know the correct answer?
By pipet, 11.00 mL of a 0.823 MM stock solution of potassium permanganate (KMnO4) was transferred to...
Questions in other subjects:


Business, 24.07.2019 13:00

History, 24.07.2019 13:00





Mathematics, 24.07.2019 13:00


Chemistry, 24.07.2019 13:00


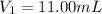
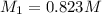
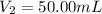
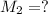
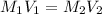 ( dilution )
( dilution )