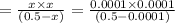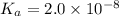Chemistry, 19.03.2020 20:40, afosburgh20
50 POINTS PLEASE HELP ASAP! I WILL MARK BRAINLIEST ONLY IF CORRECT AND CLEARLY STATED
19. Calculate the hydrogen-ion concentration [H^+] for the aqueous solution in which [OH^-] is 1 x 10^-11 mol/L. Is this solution acidic, basic, or neutral? Show your work. (3 points)
20. Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5 M solution of this acid gives a hydrogen-ion concentration of 0.000 1.M? Show your work. (3 points)
DO NOT Copy and past the work from another answer UNLESS you can clearly state all the steps. I've seen them all so I know If you copy.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, alexisdiaz365
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
Do you know the correct answer?
50 POINTS PLEASE HELP ASAP! I WILL MARK BRAINLIEST ONLY IF CORRECT AND CLEARLY STATED
1...
1...
Questions in other subjects:


Geography, 19.10.2019 15:30




Mathematics, 19.10.2019 15:30

Mathematics, 19.10.2019 15:30

Mathematics, 19.10.2019 15:30

Social Studies, 19.10.2019 15:30

Mathematics, 19.10.2019 15:30


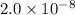 .
.![[OH^-]=1\times 10^{-11}](/tpl/images/0554/4732/291a3.png)
![pOH=-\log[OH^-]](/tpl/images/0554/4732/fe336.png)
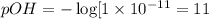
![pH=-\log[H^+]](/tpl/images/0554/4732/cf945.png)
![3=-\log[H^+]](/tpl/images/0554/4732/66d3f.png)
![[H^+]=10^{-3} M=0.001 M](/tpl/images/0554/4732/fcc5c.png)
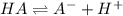
![[H^+]=x=0.0001 M](/tpl/images/0554/4732/b3468.png)
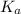 is given as:
is given as:![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0554/4732/a5cb9.png)