
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The first step is a fast reversible step and the second is a slow reaction between an oxygen atom and an ozone molecule:Step 1: O3(g) O2(g) + O(g) Fast, reversible, reactionStep 2: O3(g) + O(g) → 2O2(g) SlowWhich is the rate determining step?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, lemonsalt9378
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Do you know the correct answer?
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The f...
Questions in other subjects:

Biology, 08.02.2021 19:40

Mathematics, 08.02.2021 19:40


Mathematics, 08.02.2021 19:40

Geography, 08.02.2021 19:40


Mathematics, 08.02.2021 19:40


English, 08.02.2021 19:40

Biology, 08.02.2021 19:40


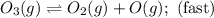
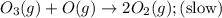
![\text{Rate}=k[O_3][O]](/tpl/images/0554/3771/ba34f.png)




