
Chemistry, 19.03.2020 19:26, austinmontgomep7foxp
The following two reactions are important in the blast furnace production of iron metal from iron ore (Fe2O3):2C(s) + O2(g) -> 2CO(g)Fe2O3 + 3CO(g) -> 2Fe + 3CO2Using these balanced reactions, how many moles of O2 are required for the production of 5.00 kg of Fe?A) 67.1 molesB) 29.8 molesC) 7.46 molesD) 89.5 molesE) 16.8 moles

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
Do you know the correct answer?
The following two reactions are important in the blast furnace production of iron metal from iron or...
Questions in other subjects:

Biology, 16.01.2021 04:10

Mathematics, 16.01.2021 04:10


Chemistry, 16.01.2021 04:10




Mathematics, 16.01.2021 04:10


Social Studies, 16.01.2021 04:10


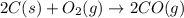
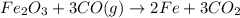
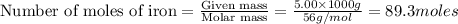
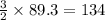 moles of carbon monoxide
moles of carbon monoxide moles of oxygen
moles of oxygen



