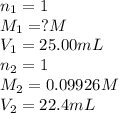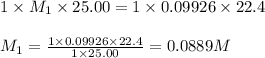Chemistry, 19.03.2020 18:34, wafflewarriormg
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and the equivalence point volume was determined by graphical means to be 22.4 mL. What is the concentration of the acetic acid?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and...
Questions in other subjects:

Mathematics, 01.04.2021 18:30





Mathematics, 01.04.2021 18:30

Mathematics, 01.04.2021 18:30

Mathematics, 01.04.2021 18:30

Chemistry, 01.04.2021 18:30


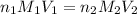
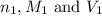 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 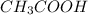
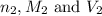 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.