Chemistry, 19.03.2020 17:08, Levantine3667
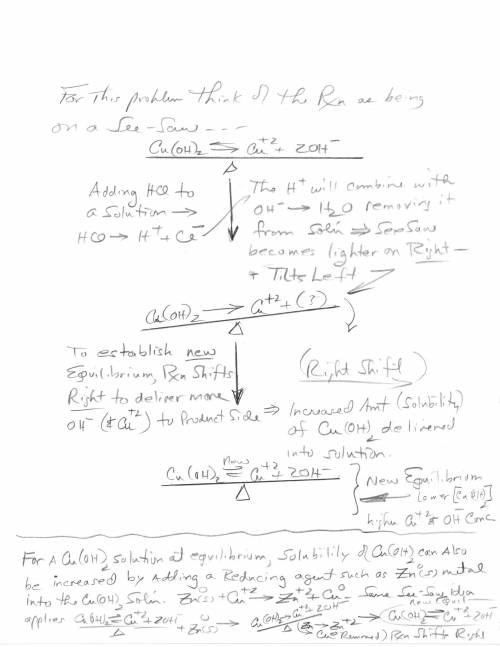
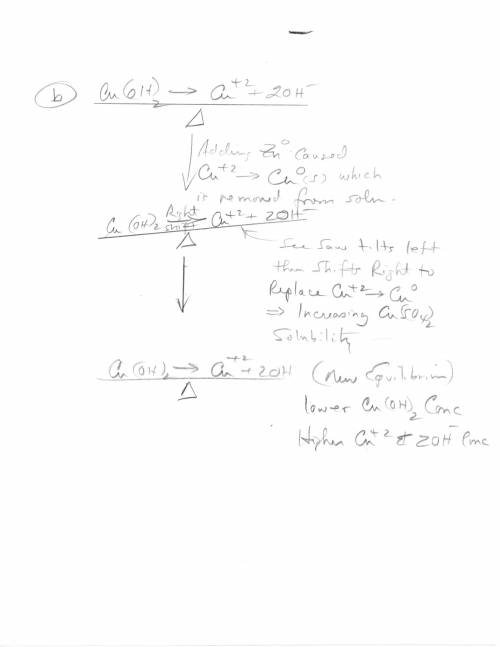
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly soluble. Cu(OH)2(s) Cu2 (aq) 2OH- (aq) a. Explain how the solubility can be increased by adding HCl to the solution. b. Explain how the concentration of copper (II) ion or of hydroxide ion can be reduced in the solution so that more of the solid copper hydroxide can be dissolved.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1


Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
Do you know the correct answer?
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly...
Questions in other subjects:

Mathematics, 15.11.2020 14:00




Mathematics, 15.11.2020 14:00


Mathematics, 15.11.2020 14:00

Physics, 15.11.2020 14:00

History, 15.11.2020 14:00

Arts, 15.11.2020 14:00