
Chemistry, 19.03.2020 17:10, studybuddy0203
Predict the effect of increasing the container volume on the amounts of each reactant and product in the following reactions: (a) CH3OH(l) <--> CH3OH(g) (b) CH4(g) + NH3(g) <--> HCN(g) + 3H2(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Do you know the correct answer?
Predict the effect of increasing the container volume on the amounts of each reactant and product in...
Questions in other subjects:

Mathematics, 11.09.2020 22:01

Biology, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Social Studies, 11.09.2020 22:01

English, 11.09.2020 22:01

English, 11.09.2020 22:01

English, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01




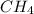 and 1 mol of
and 1 mol of 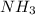 ) and in the right side we will have 4 moles (1 mol of
) and in the right side we will have 4 moles (1 mol of 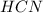 and 3 mol of
and 3 mol of 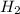 ). Therefore, the reaction will move to the right side.
). Therefore, the reaction will move to the right side.



