Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it ob...

Chemistry, 19.03.2020 17:16, jadielmatmat
Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it obeys this rate law. rate
rate= (46.6M^-1. s^-1) [H3PO4]^2
Suppose a vessel contains H3PO4 at a concentration of 0.660M. Calculate how long it takes for the concentration of H3PO$ to decrease to 20% to its natural value. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1



Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 05.10.2019 06:30



Social Studies, 05.10.2019 06:30

Physics, 05.10.2019 06:30



![Rate=k[H_3PO_4]^2](/tpl/images/0554/2402/79104.png)
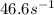
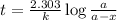

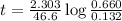
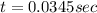
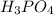 to decrease to 20% to its natural value is 0.0345 sec
to decrease to 20% to its natural value is 0.0345 sec



