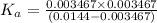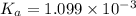Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
Do you know the correct answer?
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0144 0.0144 M solution. The pH...
Questions in other subjects:


Chemistry, 16.07.2019 21:50

English, 16.07.2019 21:50


English, 16.07.2019 21:50

Mathematics, 16.07.2019 21:50


Health, 16.07.2019 21:50

Chemistry, 16.07.2019 21:50

Mathematics, 16.07.2019 21:50


 .
.![pH=-\log[H^+]](/tpl/images/0554/0412/cf945.png)
![2.46=-\log[H^+]](/tpl/images/0554/0412/98673.png)
![[H^+]=0.003467 M](/tpl/images/0554/0412/5a2c6.png)
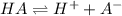
![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0554/0412/a5cb9.png)
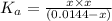
![x=[H^+]=0.003467 M](/tpl/images/0554/0412/5abc8.png)