
Chemistry, 19.03.2020 08:58, jetblackcap
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) K c = 1.80 at 250 ∘ C A 0.1846 mol sample of PCl 5 ( g ) is injected into an empty 2.55 L reaction vessel held at 250 ∘ C. Calculate the concentrations of PCl 5 ( g ) and PCl 3 ( g ) at equilibrium.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
Do you know the correct answer?
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g...
Questions in other subjects:

Mathematics, 06.07.2019 16:10




History, 06.07.2019 16:10

Biology, 06.07.2019 16:10


Geography, 06.07.2019 16:10


Mathematics, 06.07.2019 16:10


![[PCl_3]_{eq}=0.0697M\\](/tpl/images/0553/8714/e4a7f.png)
![[PCl_5]_{eq}=0.00269M](/tpl/images/0553/8714/6813e.png)
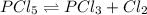
![[PCl_5]_0=\frac{0.1846mol}{2.55L}=0.0724M](/tpl/images/0553/8714/c9006.png)
![Kc=\frac{[Cl_2]_{eq}[PCl_3]_{eq}}{[PCl_5]_{eq}}](/tpl/images/0553/8714/38534.png)
 due to the reaction extent, it becomes:
due to the reaction extent, it becomes: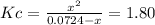
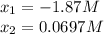
![[PCl_3]_{eq}=x=0.0697M\\](/tpl/images/0553/8714/b7f0d.png)
![[PCl_5]_{eq}=0.0724M-x=0.0724M-0.0697M=0.00269M](/tpl/images/0553/8714/fc102.png)




