Chemistry, 19.03.2020 08:57, cristianTalonzo
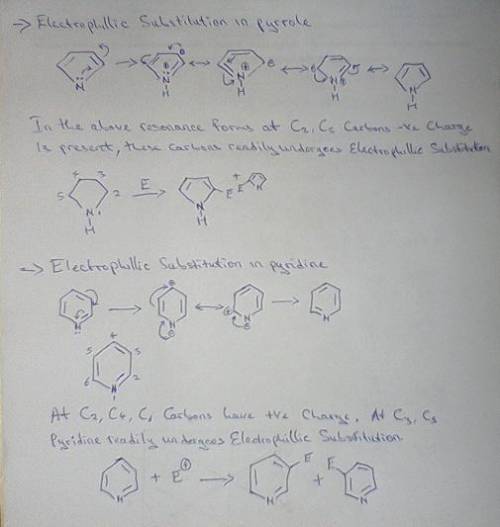
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (EAS). Using a resonance argument, predict the regiochemistry of EAS on both pyrrole and pyridine. As part of your argument, you must draw resonance structures for the arenium ion leading to each possible regiochemistry, and compare the stabilities of the resonance hybrids. In addition, make a reasoned statement about whether you think each one would react faster or slower than benzene and why.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2



Chemistry, 23.06.2019 15:40, thatlostgirl7740
Which functions of water in living systems would still be possible if water was not polar and did not form hydrogen bonds? check all that apply. climate regulation dissolving ionic compounds for biological reactions providing body support by exerting pressure on cell walls providing body support through buoyancy transport of nutrients within organisms temperature regulation in many organisms
Answers: 3
Do you know the correct answer?
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (E...
Questions in other subjects:

Biology, 05.10.2020 14:01


English, 05.10.2020 14:01


Business, 05.10.2020 14:01





Mathematics, 05.10.2020 14:01