
Chemistry, 19.03.2020 08:27, AllyJungkookie
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of solution. What is the concentration of NO3− ions in the solution? Assume that Mg(NO3)2 is the only solute in the solution. The molar mass of Mg(NO3)2 is 148.33 g/mol.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of so...
Questions in other subjects:

Health, 23.01.2021 20:30

Mathematics, 23.01.2021 20:30

English, 23.01.2021 20:30



Mathematics, 23.01.2021 20:30

Mathematics, 23.01.2021 20:30

Chemistry, 23.01.2021 20:30



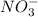 is, 2.88 M
is, 2.88 M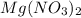 = 21.3 g
= 21.3 g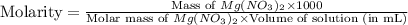
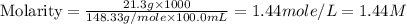
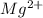 ion and 2 mole of
ion and 2 mole of 



