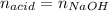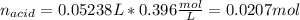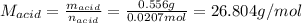Chemistry, 19.03.2020 07:52, quickestlearner6171
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 52.38 mL of 0.396 M NaOH. Calculate the molar mass of the acid, assuming it to be a monoprotic acid

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
Do you know the correct answer?
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 5...
Questions in other subjects:


Mathematics, 05.04.2021 18:50

Mathematics, 05.04.2021 18:50



Mathematics, 05.04.2021 18:50


Mathematics, 05.04.2021 18:50

English, 05.04.2021 18:50

History, 05.04.2021 18:50