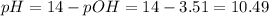Chemistry, 19.03.2020 07:20, joejoefofana
Calculate the ph of a solution containing an amphetamine concentration of 250 mg/l with amphetamine (c9h13n) is a weak base with a pkb of 4.2.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, Serenitybella
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Do you know the correct answer?
Calculate the ph of a solution containing an amphetamine concentration of 250 mg/l with amphetamine...
Questions in other subjects:


Mathematics, 26.02.2021 02:30

Chemistry, 26.02.2021 02:30

Geography, 26.02.2021 02:30


Mathematics, 26.02.2021 02:30

Mathematics, 26.02.2021 02:30

Chemistry, 26.02.2021 02:30

Mathematics, 26.02.2021 02:30

Advanced Placement (AP), 26.02.2021 02:30


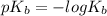
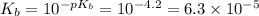
![K_b=\frac{[C_9H_{14}N][OH^-]}{[C_9H_{13}N]}\\= 6.3 \times 10^-^5](/tpl/images/0553/6976/4baa2.png)
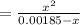
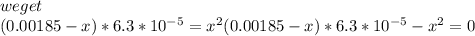
![K_{b} = \frac{[C_{9}H_{13}NH^{+}][OH^{-}]}{[C_{9}H_{13}N]}](/tpl/images/0553/6976/3720e.png)
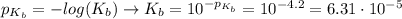
![[C_{9}H_{13}N] = \frac{250 mg}{L} \cdot \frac{1 g}{1000 mg} \cdot \frac{1 mol}{135.206 g} = 1.85 \cdot 10^{-3} mol/L](/tpl/images/0553/6976/f2089.png)
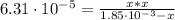
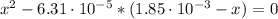 (2)
(2)![pOH = -log([OH^{-}]) = -log(0.0003116) = 3.51](/tpl/images/0553/6976/2da93.png)