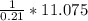A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate the molar composition of this gas on both awet and a dry basis and the ratio (mol H2O/ mol dry gas). (b) If100kg/30%excessair,(kmol/ h)? How would the answer change if the combustion were only 75% complete?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
Do you know the correct answer?
A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) Calculate t...
Questions in other subjects:

English, 06.05.2020 05:44


Chemistry, 06.05.2020 05:44


Mathematics, 06.05.2020 05:44

Health, 06.05.2020 05:44


History, 06.05.2020 05:44

Mathematics, 06.05.2020 05:44


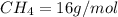
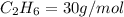
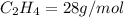
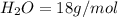
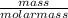
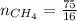
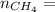 4.69 moles
4.69 moles 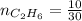
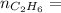 0.33 moles
0.33 moles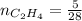
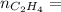 0.18 moles
0.18 moles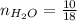
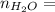 0.56 moles
0.56 moles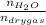
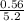




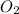 required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles
required = (2 × 4.69) + (3.5 × 0.33) + (3 × 0.18) k moles