
Chemistry, 19.03.2020 06:31, nikejenkins18
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compartment and 0.600 mole of n2(g) at 100.08c and 2.00 atm in the other compartment. these compartments are connected by a partition that transmits heat. what is the final tempera-ture in the box at thermal equilibrium? [for he(g), cv 5 12.5 j k 21mol 21 ; for n2(g), cv 5 20.7 j k 21mol 21

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
Do you know the correct answer?
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compart...
Questions in other subjects:


Mathematics, 06.01.2021 18:10


Mathematics, 06.01.2021 18:10



Chemistry, 06.01.2021 18:10




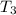 = 341.08 K
= 341.08 K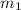 = 0.4 mole
= 0.4 mole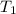 = 20.08 °c = 293.08 K
= 20.08 °c = 293.08 K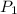 = 1 atm = 101.325 K pa
= 1 atm = 101.325 K pa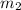 = 0.6 mole
= 0.6 mole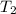 = 373.08 K
= 373.08 K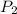 = 2 atm = 202 K pa
= 2 atm = 202 K pa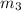 =
= 



