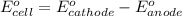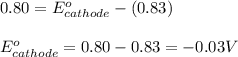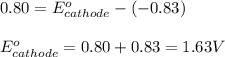Chemistry, 19.03.2020 03:59, hardwick744
A certain half-reaction has a standard reduction potential E⁰ʀᴇᴅ = 0.83 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that must provide at least 0.80V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell.
(1) Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".
(2) Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2
Do you know the correct answer?
A certain half-reaction has a standard reduction potential E⁰ʀᴇᴅ = 0.83 V. An engineer proposes usin...
Questions in other subjects:


History, 29.09.2019 01:10

Chemistry, 29.09.2019 01:10

History, 29.09.2019 01:10


Medicine, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10


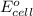 of the reaction, we use the equation:
of the reaction, we use the equation: