
When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressure calorimeter, the following reaction occurs: Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) If the temperature of the solution increases from 23.0 ∘C to 34.1 ∘C as a result of this reaction, calculate ΔH in kJ/mol of Mg. Assume that the solution has a specific heat of 4.18 J/g∘C.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1


Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
Do you know the correct answer?
When 0.243 g of Mg metal is combined with enough HCl to make 100 mL of solution in a constant-pressu...
Questions in other subjects:



Mathematics, 12.04.2021 19:30


Mathematics, 12.04.2021 19:30


Mathematics, 12.04.2021 19:30



Business, 12.04.2021 19:30


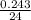 = 0.01 mol
= 0.01 mol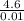 = 460 kj mol⁻¹
= 460 kj mol⁻¹



