
Chemistry, 19.03.2020 04:08, maskythegamer
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in the atmosphere occur mainly in regions with large automobile or power plant emissions. The equilibrium constant for the reaction of N2 and 02 to give NO is very small. The reaction is, however, highly endothermic, with a heat of reaction equal to +180 kJ (Equation 7). N2(g) + O2(g) 180 kJ 2NO(g) Equation 7 +
(a) Use LeChâtelier's Principle to explain why the concentration of NO at equilibrium increases when the reaction takes place at high temperatures.
(b) Use LeChâtelier's Principle to predict whether the concentration of NO at equilibrium should increase when the reaction takes place at high pressures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Do you know the correct answer?
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in th...
Questions in other subjects:

Mathematics, 13.02.2020 22:46

Mathematics, 13.02.2020 22:46


Mathematics, 13.02.2020 22:46








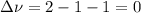

 ,
, 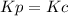 , so no effect in concentration is due to the pressure.
, so no effect in concentration is due to the pressure.




