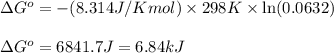Chemistry, 19.03.2020 02:55, xatziry1717
At 25 ∘ C , the equilibrium partial pressures for the reaction 3 A ( g ) + 4 B ( g ) − ⇀ ↽ − 2 C ( g ) + 3 D ( g ) were found to be P A = 5.70 atm, P B = 4.00 atm, P C = 4.22 atm, and P D = 5.52 atm. What is the standard change in Gibbs free energy of this reaction at 25 ∘ C ?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3


Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
Do you know the correct answer?
At 25 ∘ C , the equilibrium partial pressures for the reaction 3 A ( g ) + 4 B ( g ) − ⇀ ↽ − 2 C ( g...
Questions in other subjects:

Mathematics, 05.04.2021 22:50

Mathematics, 05.04.2021 22:50


Mathematics, 05.04.2021 22:50

English, 05.04.2021 22:50



Physics, 05.04.2021 22:50



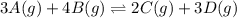
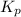 for above equation follows:
for above equation follows: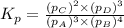
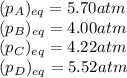
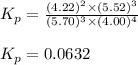
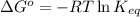
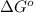 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?![25^oC=[273+25]K=298K](/tpl/images/0553/3991/0e82f.png)
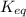 = equilibrium constant at 25°C = 0.0632
= equilibrium constant at 25°C = 0.0632