Chemistry, 19.03.2020 01:58, tristasbarker03
Calculate the volume of carbon dioxide at 20.0°C and 0.941 atm produced from the complete combustion of 4.00 kg of methane. Compare your result with the volume of CO2 produced from the complete combustion of 4.00 kg of propane (C3H8).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3


Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
Do you know the correct answer?
Calculate the volume of carbon dioxide at 20.0°C and 0.941 atm produced from the complete combustion...
Questions in other subjects:


Mathematics, 20.11.2020 22:10


History, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10


History, 20.11.2020 22:10


Medicine, 20.11.2020 22:10

Mathematics, 20.11.2020 22:10


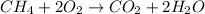
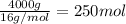
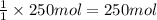 of carbon dioxide gas
of carbon dioxide gas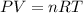 (Ideal gas equation)
(Ideal gas equation)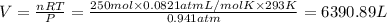
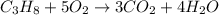
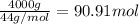
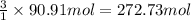 of carbon dioxide gas
of carbon dioxide gas