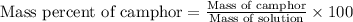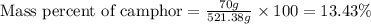Chemistry, 19.03.2020 01:36, briseisr20
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the camphor tree. Assume you dissolve 70.0 g of camphor (C10H16O) in 575 mL of ethanol, C2H5OH. Calculate the molarity, molality, mole fraction, and weight percentage of camphor in this solution. (The density of ethanol is 0.785 g/mL.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 09:00, student0724
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
Do you know the correct answer?
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the...
Questions in other subjects:


Business, 28.08.2019 15:30

Social Studies, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30

History, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30


Biology, 28.08.2019 15:30

History, 28.08.2019 15:30


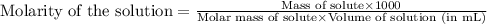
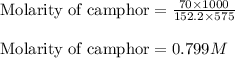
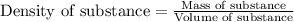

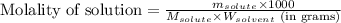
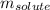 = Given mass of solute (camphor) = 70 g
= Given mass of solute (camphor) = 70 g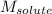 = Molar mass of solute (camphor) = 152.2 g/mol
= Molar mass of solute (camphor) = 152.2 g/mol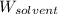 = Mass of solvent (ethanol) = 451.38 g
= Mass of solvent (ethanol) = 451.38 g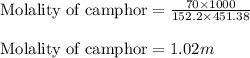
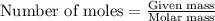 .....(1)
.....(1)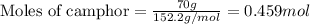
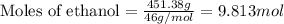
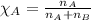
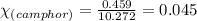 \
\