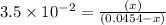Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10−2 If you start with 25.0 mLmL of a 0.909 MM solution of NaIO4NaIO4, and then dilute it with water to 500.0 mLmL, what is the concentration of H4IO−6H4IO6− at equilibrium?

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
Do you know the correct answer?
Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10...
Questions in other subjects:






Mathematics, 26.02.2022 14:00

English, 26.02.2022 14:00




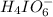 at equilibrium is, 0.00154 M
at equilibrium is, 0.00154 M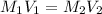
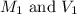 are the initial molarity and volume of
are the initial molarity and volume of 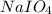 .
.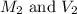 are the final molarity and volume of diluted
are the final molarity and volume of diluted 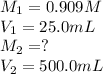
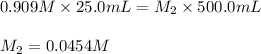
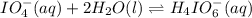
![K_c=\frac{[H_4IO_6^-]}{[IO_4^-]}](/tpl/images/0553/1589/ffca5.png)