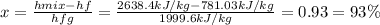Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
Do you know the correct answer?
Wet steam at 1100 kPa expands at constant enthalpy (as in a throttling process) to 101.33 kPa, where...
Questions in other subjects:



History, 08.10.2020 01:01


Computers and Technology, 08.10.2020 01:01






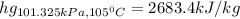
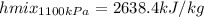
 , or fraction of the steam at the initial condition turns out:
, or fraction of the steam at the initial condition turns out: