
Chemistry, 19.03.2020 01:08, ayoismeisjjjjuan
The Haber reaction for the manufacture of ammonia is: N2 + 3H2 → 2NH3 Without doing any experiments, which of the following can you say MUST be true? Disappearance rate of H2 = 3 (Disappearance rate of N2). The reaction is first order in N2. Reaction rate = -Δ[N2]/Δt. The reaction is not an elementary reaction. Δ[H2]/Δt will have a positive value. Disappearance rate of N2 = 3 (Disappearance rate of H2). The activation energy is positive.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
Do you know the correct answer?
The Haber reaction for the manufacture of ammonia is: N2 + 3H2 → 2NH3 Without doing any experiments,...
Questions in other subjects:





English, 10.12.2020 21:00

Mathematics, 10.12.2020 21:00

History, 10.12.2020 21:00



Mathematics, 10.12.2020 21:00


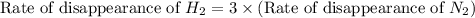
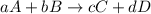
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0553/1420/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0553/1420/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0553/1420/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0553/1420/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0553/1420/d4b94.png)
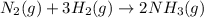
![\text{Rate of disappearance of }N_2=-\frac{d[N_2]}{dt}](/tpl/images/0553/1420/25b13.png)
![\text{Rate of disappearance of }H_2=-\frac{1}{3}\frac{d[H_2]}{dt}](/tpl/images/0553/1420/ebff2.png)
![\text{Rate of formation of }NH_3=+\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0553/1420/f55ec.png)




