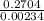Chemistry, 19.03.2020 00:28, itsmichaelhere1
Nitrogen reacts with chlorine to produce nitrogen trichloride. At equilibrium, the concentrations of each gas are as follows: 0.15 mol of nitrogen, 0.25 mol of chlorine, and 0.52 mol of nitrogen trichloride. Write the balanced equation for the reaction and calculate the equilibrium constant.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 19:40, montamonta0204
Which of the following fossils are trace fossils?
Answers: 1

Chemistry, 23.06.2019 20:50, lindsay1054
Explain what happens to a gas when it is put into a container?
Answers: 2

Chemistry, 23.06.2019 23:10, walkereddie580
Design a synthesis of 1-cyano-2-methylbicyclo[2.2.1]-2,5- heptadiene from cyclopentene and any other compounds using a diels-alder cycloaddition reaction. part 1 out of 9 choose the best option for the dienophile precursor to the target molecule.
Answers: 3

Chemistry, 24.06.2019 02:00, kappy10
An electric kettle uses electrical energy to boil water. energy from the electricity is transferred to the water, heating it up. an electric ice maker also uses electrical energy, but it freezes water to form ice. since energy can’t be created or destroyed, and water loses potential energy when it freezes to form ice, what happens to the energy put into the ice maker and the energy released by the water?
Answers: 1
Do you know the correct answer?
Nitrogen reacts with chlorine to produce nitrogen trichloride. At equilibrium, the concentrations of...
Questions in other subjects:



Mathematics, 01.02.2021 23:10

Mathematics, 01.02.2021 23:10

Mathematics, 01.02.2021 23:10

Biology, 01.02.2021 23:10

Mathematics, 01.02.2021 23:10




![\frac{[NCl_{3}^{2}]}{[N_{2}][Cl_{2}]^{3}}](/tpl/images/0552/9783/8a40e.png)
![\frac{[0.52]^{2}}{[0.15][0.25]^{3}}](/tpl/images/0552/9783/9ad1d.png)