

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
The formula for Epsom salts is MgSO4*7H2O. If 1.250g of the compound is dissolved in water, calculat...
Questions in other subjects:

History, 11.10.2021 05:30


Social Studies, 11.10.2021 05:30

Computers and Technology, 11.10.2021 05:30

Mathematics, 11.10.2021 05:30

Chemistry, 11.10.2021 05:30

Mathematics, 11.10.2021 05:30

English, 11.10.2021 05:30


Mathematics, 11.10.2021 05:30



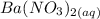 to precipitate barium sulfate.
to precipitate barium sulfate.



