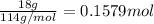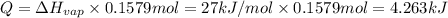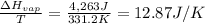Chemistry, 19.03.2020 00:24, hannahkharel2
Calculate the entropy change (J/K) for the vaporization of 18 g of a hydrocarbon (114 g/mole]), at its boiling point of 58.2°C. The enthalpy of vaporization of this hydrocarbon is 27 kJ/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, velazquez2974
Covalent bonds are formed between metals and boiling points true or false
Answers: 2


Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
Do you know the correct answer?
Calculate the entropy change (J/K) for the vaporization of 18 g of a hydrocarbon (114 g/mole]), at i...
Questions in other subjects:

Mathematics, 31.10.2020 02:40

Spanish, 31.10.2020 02:40

Biology, 31.10.2020 02:40

Social Studies, 31.10.2020 02:40

Mathematics, 31.10.2020 02:40

Geography, 31.10.2020 02:40

Business, 31.10.2020 02:40

Social Studies, 31.10.2020 02:40

Geography, 31.10.2020 02:40

English, 31.10.2020 02:40