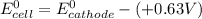A certain half-reaction has a standard reduction potential E⁰red = +0.63V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 1.30V of electrical power. The cell will operate under standard conditions.
(a) Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".
(b) Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no upper limit, write "no".

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
Do you know the correct answer?
A certain half-reaction has a standard reduction potential E⁰red = +0.63V. An engineer proposes usin...
Questions in other subjects:

Mathematics, 07.02.2021 06:40


History, 07.02.2021 06:40

Mathematics, 07.02.2021 06:40



Mathematics, 07.02.2021 06:40


Mathematics, 07.02.2021 06:40



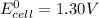
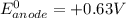
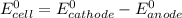
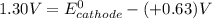
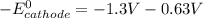
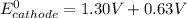
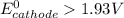 ; then
; then 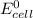 > 1.30 V
> 1.30 V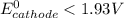 ; then
; then 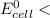 1.30 V
1.30 V