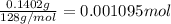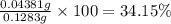Chemistry, 18.03.2020 21:26, Carlosanddana123
You are given a sample of limestone, which is mostly CaCO3, to determine the mass percentage of Ca in the rock. You dissolve the limestone in hydrochloric acid, which gives a solution of calcium chloride. Then you precipitate the calcium ion in solution by adding sodium oxalate, Na2C2O4. The precipitate is calcium oxalate, CaC2O4. You find that a sample of limestone weighing 128.3 mg gives 140.2 mg of CaC2O4. What is the mass percentage of calcium in the limestone

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
Do you know the correct answer?
You are given a sample of limestone, which is mostly CaCO3, to determine the mass percentage of Ca i...
Questions in other subjects:




Advanced Placement (AP), 30.10.2020 22:30

Mathematics, 30.10.2020 22:30

Physics, 30.10.2020 22:30


Mathematics, 30.10.2020 22:30