
Chemistry, 18.03.2020 18:55, Thelazysandwich
Based on the acid strength of H2SO3 and H2SeO3, will the equilibrium constant of H2SO3(aq)+HSeO3−(aq)⇌H2SeO3(aq)+HSO 3−(aq) be (Kc> 1) or (Kc< 1)? Group of answer choices Kc< 1, because H2SO3 is a stronger acid than H2SeO3 Kc< 1, because H2SO3 is a weaker acid than H2SeO3 Kc> 1, because H2SO3 is a weaker acid than H2SeO3 Kc> 1, because H2SO3 is a stronger acid than H2SeO3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Do you know the correct answer?
Based on the acid strength of H2SO3 and H2SeO3, will the equilibrium constant of H2SO3(aq)+HSeO3−(aq...
Questions in other subjects:

Spanish, 22.01.2021 20:50


Mathematics, 22.01.2021 20:50


Mathematics, 22.01.2021 20:50

Chemistry, 22.01.2021 20:50

English, 22.01.2021 20:50

Spanish, 22.01.2021 20:50



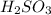 is a stronger acid than
is a stronger acid than 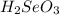

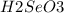 . Additionally, if we have his behavior the reaction would be displaced to the right side due to the strong acid (the strong acid is placed in the reactives side), so we will have a K greater than 1.
. Additionally, if we have his behavior the reaction would be displaced to the right side due to the strong acid (the strong acid is placed in the reactives side), so we will have a K greater than 1. 




