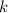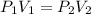A certain amount of chlorine gas was placed inside a cylinder with a movable piston at one end. The initial volume was 3.00 L and the initial pressure of chlorine was 1.85 atm . The piston was pushed down to change the volume to 1.00 L. Calculate the final pressure of the gas if the temperature and number of moles of chlorine remain constant

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
Do you know the correct answer?
A certain amount of chlorine gas was placed inside a cylinder with a movable piston at one end. The...
Questions in other subjects:






Mathematics, 23.03.2021 16:30


Mathematics, 23.03.2021 16:30


English, 23.03.2021 16:30


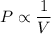
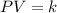 Pressure multiplied by volume equals some constant
Pressure multiplied by volume equals some constant