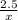2. How many grams of Potassium Chloride, KCl, are produced if 25.0 g of Potassium Chlorate, KClO3, decompose?
2KClO3 → 2KCl + 3O2
3. What is the moles of H2 gas produced when 100.0 grams of Na is added to the reaction?
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
4. How many moles of Hydrogen, H2, are needed to react with 2.5 moles of Nitrogen, N2?
N2 + 3H2 → 2NH3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
2. How many grams of Potassium Chloride, KCl, are produced if 25.0 g of Potassium Chlorate, KClO3, d...
Questions in other subjects:



Social Studies, 20.07.2019 19:30


Chemistry, 20.07.2019 19:30


Social Studies, 20.07.2019 19:30

Biology, 20.07.2019 19:30



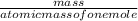
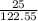
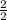 =
= 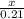
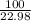
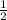 =
= 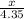
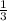 =
=