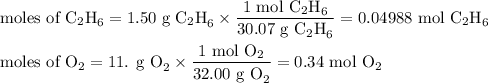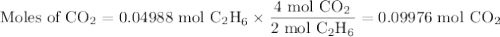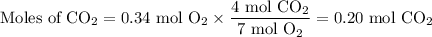Chemistry, 18.03.2020 00:42, bravomichelle75
Gaseous ethane (CH, CH,) will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO) and gaseous water (H2O). Suppose 1.50 g of
ethane is mixed with 11. g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Round your answer to 2
significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 13:50, isaac7454
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1
Do you know the correct answer?
Gaseous ethane (CH, CH,) will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO)...
Questions in other subjects:


Mathematics, 25.04.2021 14:00




Mathematics, 25.04.2021 14:00

Mathematics, 25.04.2021 14:00

Computers and Technology, 25.04.2021 14:00

Spanish, 25.04.2021 14:00