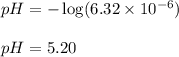The initial concentration of ammonia is 0.14 M and the pH of the solution at equivalence point is 5.20
Explanation:
To calculate the number of moles for given molarity, we use the equation:
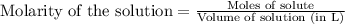 .....(1)
.....(1)
Molarity of HCl solution = 0.164 M
Volume of solution = 23.8 mL = 0.0238 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
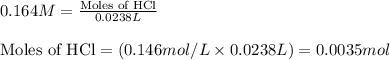
The chemical equation for the reaction of ammonia and HCl follows:
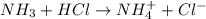
By Stoichiometry of the reaction:
1 mole of HCl reacts with 1 mole of ammonia
So, 0.0035 moles of HCl will react with = 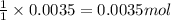 of ammonia
of ammonia
Calculating the initial concentration of ammonia by using equation 1:
Moles of ammonia = 0.0035 moles
Volume of solution = 25 mL = 0.025 L
Putting values in equation 1, we get:
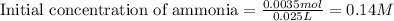
By Stoichiometry of the reaction:
1 mole of ammonia produces 1 mole of ammonium ion
So, 0.0035 moles of ammonia will react with = 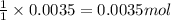 of ammonium ion
of ammonium ion
Calculating the concentration of ammonium ion by using equation 1:
Moles of ammonium ion = 0.0035 moles
Volume of solution = [23.8 + 25] mL = 48.8 mL = 0.0488 L
Putting values in equation 1, we get:
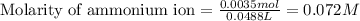
To calculate the acid dissociation constant for the given base dissociation constant, we use the equation:
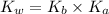
where,
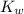 = Ionic product of water =
= Ionic product of water = 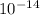
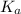 = Acid dissociation constant
= Acid dissociation constant
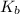 = Base dissociation constant =
= Base dissociation constant = 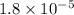
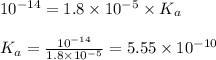
The chemical equation for the dissociation of ammonium ion follows:
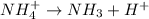
The expression of 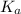 for above equation follows:
for above equation follows:
![K_a=\frac{[NH_3][H^+]}{[NH_4^+]}](/tpl/images/0551/3928/47aaa.png)
We know that:
![[NH_3]=[H^+]=x](/tpl/images/0551/3928/ae0a0.png)
![[NH_4^+]=0.072M](/tpl/images/0551/3928/583ca.png)
Putting values in above expression, we get:
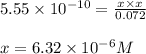
To calculate the pH concentration, we use the equation:
![pH=-\log[H^+]](/tpl/images/0551/3928/cf945.png)
We are given:
![[H^+]=6.32\times 10^{--6}M](/tpl/images/0551/3928/81536.png)
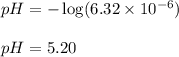
Hence, the initial concentration of ammonia is 0.14 M and the pH of the solution at equivalence point is 5.20
















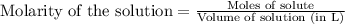 .....(1)
.....(1)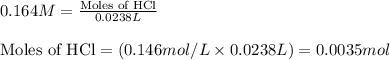
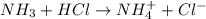
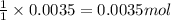 of ammonia
of ammonia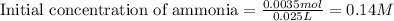
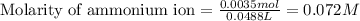
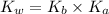
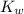 = Ionic product of water =
= Ionic product of water = 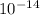
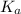 = Acid dissociation constant
= Acid dissociation constant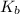 = Base dissociation constant =
= Base dissociation constant = 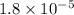
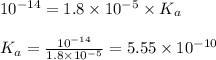
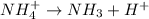
![K_a=\frac{[NH_3][H^+]}{[NH_4^+]}](/tpl/images/0551/3928/47aaa.png)
![[NH_3]=[H^+]=x](/tpl/images/0551/3928/ae0a0.png)
![[NH_4^+]=0.072M](/tpl/images/0551/3928/583ca.png)
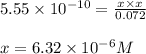
![pH=-\log[H^+]](/tpl/images/0551/3928/cf945.png)
![[H^+]=6.32\times 10^{--6}M](/tpl/images/0551/3928/81536.png)