
Find the pH of each mixture of acids. Acid Ionization Constants (Ka) for Some Monoprotic Weak Acids at 25 ∘C Acid Formula Ka Benzoic acid HC7H5O2 6.5×10−5 Hydrofluoric acid HF 6.8×10−4 Phenol HC6H5O 1.3×10−10 Formic acid HCHO2 1.8×10−4 Hypochlorous acid HClO 2.9×10−8

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2


Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
Find the pH of each mixture of acids. Acid Ionization Constants (Ka) for Some Monoprotic Weak Acids...
Questions in other subjects:

Mathematics, 02.03.2021 20:20

Mathematics, 02.03.2021 20:20

Mathematics, 02.03.2021 20:20

Mathematics, 02.03.2021 20:20

Business, 02.03.2021 20:20



History, 02.03.2021 20:20


Mathematics, 02.03.2021 20:20


![[H^+] = \sqrt{K_2C}](/tpl/images/0551/1514/821c9.png)
![[H^+] = \sqrt{6.5*10^{-5}*0.185M}](/tpl/images/0551/1514/28253.png)
![[H^+] =0.0035M](/tpl/images/0551/1514/0669f.png)
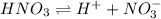
![[H^+]](/tpl/images/0551/1514/07acb.png) = 0.070 M
= 0.070 M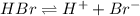
![[H^+] = 0.025 M](/tpl/images/0551/1514/a4539.png)
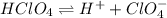
![[H^+] = 0.020 M](/tpl/images/0551/1514/f72cd.png)
![[H^+] = 0.020 M + 0.025 M](/tpl/images/0551/1514/f46a7.png)
![[H^+] = 0.045 M](/tpl/images/0551/1514/4bd75.png)
![[H^+] = \sqrt{K_{a1}C_1+K_{a2}C_2}](/tpl/images/0551/1514/9f22b.png)
![[H^+] = \sqrt{6.8*10^{-4}*0.095 +1,3*10^{-10}*0.230](/tpl/images/0551/1514/a064b.png)
![[H^+] = 0.008037 M](/tpl/images/0551/1514/33641.png)




